>BACTERIOPHAGES (generally called PHAGES)
Introduction by Pida Ripley, Founder, CombatAMR
I developed an enduring interest in the development and use of phage therapy over two decades ago when I was implementing humanitarian and development programmes in the collapsed states of the former USSR. In the Caucasus I became aware of the bacteriophage therapy work undertaken by the Eliava Institute of Bacteriophages, Microbiology and Virology in Tbilisi, as many of my staff members used phage therapy rather than antibiotics. In 2006, when undergoing a total knee replacement and having a long history of multiple serious infections, I made sure I had the full details of the Institute - just in case I ended up with a drug resistant infection in my knee. Thankfully my notes were unnecessary!
>Video: Eliava Institute of Bacteriophages Tbilisi Georgia
BACTERIOPHAGES ARE SPECIALIST VIRUSES THAT ONLY KILL BACTERIA
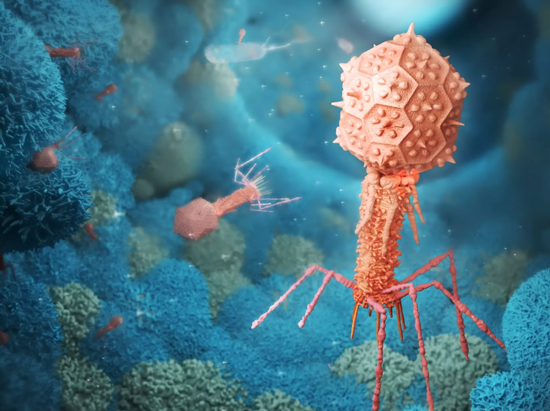
A bacteriophage is a virus that infects and replicates within a specific bacterium. The term bacteriophage is derived from "bacteria" and the Greek: (phagein), "to devour". A specific class of viruses able to infect and destroy bacteria, they are among the most common and diverse entities in the biosphere. Fortunately phages offer no threat to humans. Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have relatively simple or elaborate structures. Their genomes may encode as few as four genes, and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into the cytoplasm of the bacteria.
They are intrinsically narrow spectrum agents and are restricted to a single bacterial species. Widely distributed in locations populated by bacterial hosts, such as soil or the intestines of animals, one of the most dense natural source for phages and other viruses is sea water; up to 70% of marine bacteria may be infected by phages.
Phage therapy development and interest began over a century ago. In 1915, British bacteriologist Frederick Twort published AN INVESTIGATION ON THE NATURE OF ULTRA-MICROSCOPIC VIRUSES in the Lancet. It was the first report of viruses that infect bacteria, replicate there and kill the cells. (F.W.Twort L.R.C.P. LOND., M.R.C.S.) Published: Lancet 186, 1241–1243 (1915) December 04, 1915. DOI:https://doi.org/10.1016/S0140-6736(01)20383-3)

Phage therapy development and interest began over a century ago. Félix d'Hérelle pioneered work on phages, the 'devours of bacteria' and in 1917 he finally concluded that the bacteriophage is a submicroscopic virus, parasitic on susceptible bacteria, with transmissible lysis of bacteria. He was among the first scientists to successfully treat bacterial infections with phages. Felix d'Hurelle:The bacteriophage and its behaviour.1914 Baltimore. Williams & Wilkins Co.
Before the discovery of penicillin in 1928, phage therapy was tried extensively in the West in the 1920s and 1930s. Despite the desperate clinical battle against infections in the pre-antibiotic era, phage therapy was largely undeveloped by the time penicillin was made widely available in the 1940s. The easy to use and highly effective broad spectrum penicillin antibiotic became known as the 'miracle drug' - saving millions of lives and interest in phage treatment waned.
However, phage therapy continued being developed and used for over 90 years as an alternative to antibiotics and a treatment of choice in the former Soviet Union and Central Europe, as well as in France. The increasing global threat posed by antimicrobial resistance (AMR) is now propelling renewed interest in bacteriophages.
A problem of this underdeveloped therapy is that very few controlled, well-designed clinical trials have tested its efficacy and safety. The anecdotal only evidence of its success has severely limited wider global implementation, despite successful outcomes for 'last resort' patients and continuing support from populations able to access phage therapy. Validation of phage treatment for human infectious diseases must be obtained by conducting robust clinical trials. As antimicrobial resistance increases, with no new antibiotics in the pipeline, more governments are beginning to allocate funding for further research on phage therapy.
THE GLOBAL DISCOVERY GAP
During the past three decades the World Health Organisation (WHO) issued multiple warnings about increasing drug resistance, but very little action was taken by governments or the pharmaceutical companies. Many pharmaceutical companies have closed their antibiotic research and development programmes due to irrecoverable high costs involved in seeking new antibiotics. As our 'last line' antibiotics are beginning to be ineffective against multi-drug resistant bacteria, belatedly there is realisation that there are no new antibiotics in the pipeline.
There is a global discovery gap during which we all will face increased life-threatening rsk. Clinical trials take many years to confirm safety and efficacy and even if potential new antibiotics are discovered, the development process will take 5-15 years before there is any availability. This means we have to take all possible steps to protect the antibiotics that still work by limiting their use to when absolutely necessary only. Infections can be avoided by improving personal, food and animal hygiene standards. One of the most effective preventative acts all can take is washing and soaping our hands and nails thoroughly - for 20 seconds - not the average 5-6 seconds quick rinse!
LATEST PHAGE NEWS
>Directed Evolution of a Mycobacteriophage
-25 April 2019. by María Cebriá-Mendoza,Rafael Sanjuán andPilar Domingo-Calap Antibiotics 2019, 8(2), 46; https://doi.org/10.3390/antibiotics8020046
Abstract Bacteriophages represent an alternative strategy to combat pathogenic bacteria. Currently, Mycobacterium tuberculosis infections constitute a major public health problem due to extensive antibiotic resistance in some strains. Using a non-pathogenic species of the same genus as an experimental model, Mycobacterium smegmatis, here we have set up a basic methodology for mycobacteriophage growth and we have explored directed evolution as a tool for increasing phage infectivity and lytic activity. We demonstrate mycobacteriophage adaptation to its host under different conditions. Directed evolution could be used for the development of future phage therapy applications against mycobacteria. Full article
>Monash biologist awarded over $130,000 to investigate bacteriophage therapy
https://www.monash.edu/science/news/current/monash-biologist-awarded-over-$130,000-to-investigate-bacteriophage-therapy
02 November 2018
Research on bacteriophages has won Monash University biologist Dr Jeremy J. Barr, a Ramaciotti Award for Biomedical Research with a grant of $137,534. The Clive and Vera Ramaciotti Foundation, this week announced $784,731 in funding to seven biomedical researchers.
Dr Barr is the Head of the Bacteriophage Biology Research Group at the Monash University School of Biological Sciences. Dr Barr's lab studies bacteriophages -- specialist viruses that only infect and kill bacteria -- and investigates their role and function within the human body.
PHAGE THERAPY SAVES A LIFE AND INITIATES THE FIRST PHAGE THERAPY CENTRE IN THE US
The first known person in the United States to successfully undergo intravenous bacteriophage (phage) therapy was Tom Patterson, PhD , professor of psychiatry at UC San Diego School of Medicine. He had contracted a life-threatening infection with a multidrug-resistant strain of Acinetobacter baumannii, an opportunistic and often deadly bacteria, while vacationing in Egypt in November 2015. Patterson was eventually transported to UC San Diego Health where, with emergency approval from the Food and Drug Administration (FDA), he was treated intravenously with an experimental phage cocktail that specifically targeted A. baumannii. He began improving almost immediately, emerging from a months-long coma.
Patterson's experience opened a fresh avenue of research aimed at finding alternatives to traditional antibiotics, amidst the growing problem of antimicrobial resistance. In an effort led by Patterson's wife, Steffanie Strathdee, PhD, Associate Dean of Global Health Sciences at UC San Diego School of Medicine, and Robert "Chip" Schooley, MD, professor of medicine and an infectious disease specialist at UC San Diego Health, five patients at UC San Diego Health have now been treated with phages. One of these patients had a years-long chronic infection that was successfully cleared, allowing him to undergo life-saving heart transplant surgery. In all cases, the phage treatments were considered experimental and required emergency approval by the FDA.
In June 2018, to further advance this work, UC San Diego School of Medicine launched the interdisciplinary Center for Innovative Phage Applications and Therapeutics (IPATH) with a three-year, $1.2 million grant from UC San Diego Chancellor Pradeep Khosla. Co-directed by Strathdee and Schooley, it is the first such Center in North America.
Learn more about Bacteriophage Therapy at UC San Diego Health:
https://health.ucsd.edu/news/topics/phage-therapy/Pages/default.aspx
https://health.ucsd.edu/news/topics/phage-therapy/Pages/Phage-101.aspx
For the full story view the video and the Tedx Talk by Steffanie Strathdee, PhD below.
>Phage Treatment Saves A Life
25 April 2017. UC San Diego Health
Scientists and physicians at University of California San Diego School of Medicine, working with colleagues at the U.S. Navy Medical Research Center (NMRC), Texas A&M University, a San Diego-based biotech and elsewhere, have successfully used an experimental therapy involving bacteriophages.
Bacteriophages are viruses that target and consume specific strains of bacteria to treat a patient near death from a multidrug-resistant bacterium.
>Video: How Sewage Saved My Husband's Life from a Superbug - Steffanie Strathdee | TEDxNashville
Published on 17 Oct 2017.
https://www.youtube.com/watch?v=AbAZU8FqzX4
Strathdee was recently credited with saving her husband's life from a deadly superbug infection using bacteriophages –viruses that attack bacteria. The case, which involved cooperation from three universities, the U.S. Navy and researchers across the globe, shows how phage therapy is a future weapon against multi-drug resistant bacterial infections which are expected to kill 10 million people per year by 2050.
MORE VIDEOS ABOUT BACTERIOPHAGES
>The Deadliest Beings on the Planet
Published on 13 May 2018. A war has been raging for billions of years, killing trillions every single day, while we don't even notice.
>What is BACTERIOPHAGE?
Published on 23 Sep 2016
Video explains how they work.
Revaz Adamia - Director of the Eliava Institute Tbilisi: Applied Aspects of Bacteriophages
Published 8 Mar 2012. Scientist, diplomat and politician, Revaz Adamia, describes the use of bacteriophages in medicine and his institute's journey through the Soviet collapse to the present. He is the Director of the G.Eliava Institute of Bacteriophages, Microbiology and Virology. Parallel to his phage research and work, Revaz had a long political career, as an MP and then appointed Georgia's Ambassador to the UN.
>Phages:Nature's Ninjas in the battle against Superbugs | Heather Hendrickson | TEDxTauranga
Published 6 Oct 2016. Heather Hendrickson is a Phage Hunter and an Evolutionary Biologist. In a world where bacteria are increasingly resistant to antibiotics she investigates bacterial genomes to understand how they evolve and exchange information, a process known as Horizontal Gene Transfer. She hunts for new bacteriophages to isolate and study in a continual search for alternative treatments to bacterial diseases.
>Bacteriophage Expert Dr. Alexander Sulakvelidze
Published 21 August 2015.
https://www.youtube.com/watch?v=SALlGDkfkL4
Dr. Sulakvelidze, a renowned authority in the field of bacteriophage therapy, shares his views on the role of bacteriophages in combating antimicrobial resistance - AMR.
>The Secret Soviet Virus That Helps Kill Bacteria
Published on 11 Dec 2016
https://youtu.be/jTwEVK7TMWI
Antibiotic resistance is threatening to render antibiotics useless, but scientists may have found their answer in bacteria-eating viruses.
>What Are Bacteriophages & How Do Bacteriophage / Phage Viruses Work? (With Animation)
Published 29 December 2016. https://youtu.be/i9L-RoQ1frc
Bacteriophages are viruses that infect bacteria cells and they are the most abundant biological entities on the planet, estimated at 10^31 in population. Dr Jan Mast says "I have had the pleasure of working with and researching these phages, and have discovered 3 novel phages on my own. They're great for learning about genomics and bioinformatics because of their small genomes". Dr. Jan Mast, CODA, Groeselenberg, Ukkel, Brussel.
>Dr. Tim Lu - Biofilms and Phage Therapy
Published on 26 Apr 2010. https://www.youtube.com/watch?v=3eQhNyX7DRQ
This 11 minute film is excerpted from an interview with Dr. Tim Lu, who is an expert in characterizing & eliminating biofilms with phage therapy. He offers some insightful ways to describe complex biofilms and their connection to antibiotic resistance. Interview excerpts & videos with Bacterial Biofilm Experts (doctors & researchers):
http://www.biofilmcommunity.org/
